Topic 5: Chemical Equations
a) What is a chemical reaction?
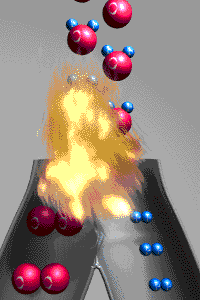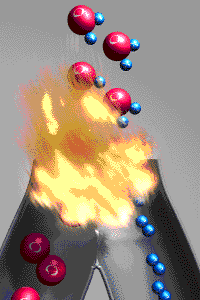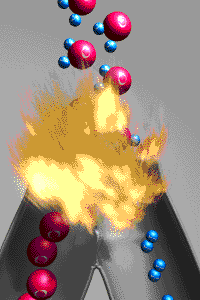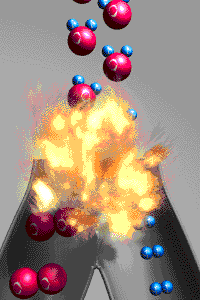
A chemical reactions is a reaction that involves
rearranging atoms from the reactants that make a new substance which are called products. To prove that a
change was a chemical change is to demonstrate the new
substances produced have different properties from those of the reactants. An exothermic reaction
releases energy to the environment because the bonds
holding the products together are stronger than the bonds holding the reactants together.
b) How are reaction written?
A formula equation uses atomic symbols to describe
the rearrangement of atoms of the reactants to form
the products in a chemical reaction. The number,
mass, types of atoms remain the same on both sides of
balanced equation. Coefficients indicate the amount
of reactants and products that can be rearranged to
balance a chemical equation. The subscripts within a
chemical formula can never be changed.
 A Simple SN2 Reaction
A Simple SN2 Reaction
CH3Br + Cl ---> CH3Cl + Br
c) How can reactions be used?
The five basic types of chemical reactions are:
combustion, synthesis, decomposition, single and
double-displacement. Polymerization reactions are
synthesis reactions that start to build molecules to
from many repeated units. Activity series assume
single-displacement reactions that happened. In which
any element on the list will displace those below its
compound. Double-displacement reactions can be
represented by net ionic equations in which show only
the ions that are changed by the reaction.