Topic 10: Acids and Bases
a) What are acids and bases?
Acid: A class of compounds whose water solutions taste sour, turn blue litmus to red, and react with bases to form salt.
Base: A class of compounds that taste bitter, feel slippery in water solution, turn red litmus to blue, and react with acids to form salts.
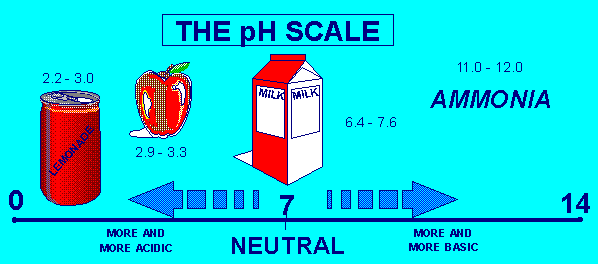
SOME EXAMPLES THAT ARE ACID OR BASE.
|
|
|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
b) How are weak acids and bases compared?

Acid and bases normally go in a scale from 1 to 14. 1 through 6 is considered acid and 8 through 14 is considered base. The number 7 is considered neutral in a pH scale. The equilibrium constant for the reaction of an aqueous weak acid with water is known as the acid-dissociation constant. Consider the equilibrium established by acetic acid in vinegar. Something like Lemon Juice
c) What are the biochemical roles of some acids and bases? (Acids and bases in the body)
Amino acids, nucleic acids, and fatty acids are biological molecules that function within the pH range of living systems. Buffers maintain pH living systems. You have acids in your body. A really strong acid is Hydrochloric acid and that is in your stomach. The acid is used to break down the food. Some times you eat too much acidic food and you get like a heart burn of gassy stomach. Well then you need to take a supplement to balance the pH level in your body.