 The Red and Yellow
are the potassium forming a chemical bond with the water.
The Red and Yellow
are the potassium forming a chemical bond with the water.
Topic 4: Ionic and Covalent Compounds
a) Why do atoms form bonds? (Energy and stability)
STABILITY:
The best place to start is examining all those atoms that generally do not form bonds. Noble Gases are located in Group 18. They have one thing in common and that is chemically. Though they are very unreactive. They exist as individual atoms and they rarely form chemical bonds. Most other elements behave very differently from the noble gases. Potassium, for example, is a very reactive metal. Potassium reacts violently with a variety of substances, including the oxygen present in air. Potassium also reacts violently in water. One product of this reaction is a compound dissolved in the beaker, which more chemically stable than the potassium metal; unlike the original potassium metal, it does not react with water or air. The forces of attraction that held the atoms in the water molecules and the atoms in the potassium metal together are broken, and the atoms of each are attracted in new ways to form new substances that are chemically more stable. These forces of attraction are called chemical bonds.
Picture:
Of potassium mixed with water. Forming
a chemical bond.
 The Red and Yellow
are the potassium forming a chemical bond with the water.
The Red and Yellow
are the potassium forming a chemical bond with the water.
Energy:
Stable Octet:
is a main-block elements form bonds by rearranging electrons so that each
atom has a stable octet in its out almost energy level.
When atoms gain stability by forming chemical bonds, keep in bind that they do lose something, ENERGY. When two atoms form a chemical bond, energy is released. When two individual potassium atoms lose electron and attain a stable octet in the reaction with water; they release some energy in the form of heat and light, and a lower potential energy level is attained.
b) How do ionic and covalent bonds compare? (Comparing bond types and properties)
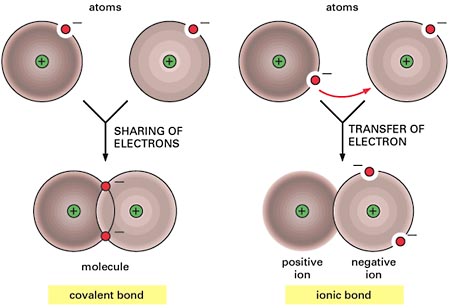
Atoms can attain a more stable arrangement of electrons in their outermost shell by interacting with one another. An ionic bond is formed when electrons are transferred from one atom to the other. A covalent bond is formed when electrons are shared between atoms. The two cases shown represent extremes; often, covalent bonds form with a partial transfer (unequal sharing of electrons), resulting in a polar covalent bond.
c) How do you name salts? (Naming ions and ionic compounds.)
Chemists use a system to write and to name the ions and the salts they form. This system is based on the chemical symbols listed in the periodic table. By using the system, chemists have a uniform standard to represent chemical compounds. The system makes communication easier, saves time, and simplifies calculations.
Ionic compounds are named by joining cation and anion names. The subscripts in the formula for an ionic compound indicates the lowest electrically neutral whole-number ration of cations to anions. Ionic compounds consisting of two elements are known as binary compounds. When naming binary compounds you have to write the cation first. For example, a barium ion (Ba2+) and an oxide ion (O2-) composed to make barium oxide.
d) What is a platonic ion? (Naming ionic compounds and writing ionic compound formulas)
A polyatomic ion is two or more atoms bonded together and functioning as a single unit. Parentheses are used to group polyatomic ions in a chemical formula with a subscript. For example, Ca(H2PO4)2 can be used to decipher if you remember that polyatomic ions act in a unit. There for , to show more than one polyatomic ion, parentheses are used.
e) How are molecules specified?
A Lewis structure models the valence electron arrangement in a molecule. In most molecules, each atom is surrounded by an octet of electrons. Some molecules have more than one valid Lewis Structure. Molecules are named using either prefixes or Roman numerals.
Formulas are represented by Empirical formulas. Empirical formulas show the smallest whole number ratio of atoms. Molecular formulas are multiples of empirical formulas. Structural formulas show the spatial arrangement of atoms in molecules.
You can tell the shape of a molecule by the VSEPR theory. The VSSEPR theory states that electron pairs are as far apart as possible. This theory can be used to predict the molecular shape.