Topic 12: Nuclear Chemistry
a) Why are come atomic nuclei unstable?
Their repulsive forces between there like charges,
protons cluster tightly together within the nucleus.
Strong forces in the nucleus overwhelms the repulsive
forces of the protons that holds protons and neutrons
together. A small percentage of atoms have nuclei
that are unstable and subject to a nuclear change
called transmutation, which results in the formation
of a new element. Atoms that exhibit this behavior
are called radioactive. Stability of the nucleus is
dependent on the neutron-proton ratio. Stable atoms
form a pattern called the band of stability. The
amount of energy released when a nucleus is formed is
called binding energy. Binding energy can be
calculated by applying Einsteinís equation, E=mc2.
b) What kinds of nuclear change occur?
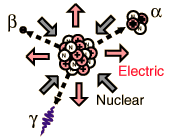
Radioactivity is composed of alpha and beta
particles, gamma rays, and positron emission.
Artificial transmutation occurs in laboratories in
machines, as particle accelerators. Fission process
makes the nucleus splits into two smaller nuclei,
releasing large quantities of energy. Fusion come
into small nuclei to combine into a larger nucleus,
releasing large quantities of energy.
Half-life is the time that is required for Ĺ of the
mass of a radioactive substance to decay. The
half-life of the carbon-14 isotope can be used to date
organic material that is up to 20,000 years old.
Other radioactive isotopes are used to find
information on mineral or ancient rock formations. To
measure the effects of radiation is called rems.
c) How is nuclear chemistry used?
The time required for one
half of the mass of a radio active substance to
decay is what half-life is. The half-life of the carbon-14 can be used to
date organic material up to the age of 20,000 years old. The effects of
radiation are measured normally with units called rems. Other isotopes are used
to date more ancient rock and mineral foundation when radioactive