Topic 2: Atomic Structure
a) How are elements organized?

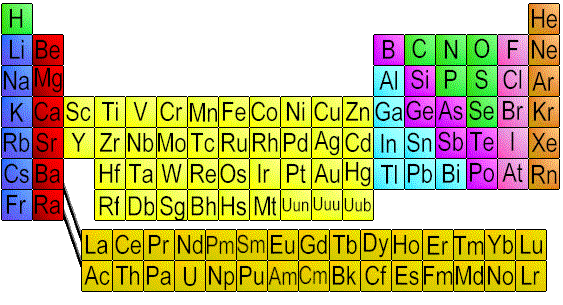
Color coated to tell the different metals appart.
The periodic table is arranged so that
elements
with similar properties fall within the same group.
In the periodic law, the properties of elements by
their atomic numbers. Elements are ordered from left
to right by increasing atomic number. The elements
with similar properties are grouped vertically in
families. Some elements have similar characteristic
properties because they have similar electron
configurations.
b) What is the basic structure of an atom?
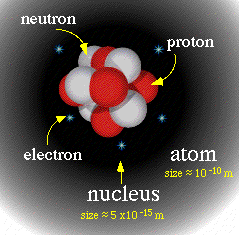
Three laws support the existence of atoms: The
law
of definite composition, conservation of mass, and
multiple proportion. Also atoms consist of particles
that have positive and negative charges. Positive
charges are confined to central nucleus that accounts
for most of the atoms mass that has the radius 10,000
times smaller then a whole atom. The nucleus also
consist and contains neutrons, and particles with no
charges. The electron, particles that have a negative
charge, and a mass that has 2,000 times smaller than
the mass of hydrogen, traveled about in the nucleus.
Discrete amounts of energy are released or absorbed
when electrons move from one energy level to another.
Quantum theory describes the wave nature of electrons.
c) How do the structures of atom differ?
The atomic number is the number of protons
contained in each atom of that element. The mass
number is the total number of protons and neutrons in
an atom. A unique set of four quantum numbers
describes each electron in an atom. The Paula
exclusion principle states that no two electrons can
have the same four quantum numbers.